RESEARCH
We are interested in the biological mechanisms of action of novel physical methods, such as ultrashort electric fields and a UV laser microbeam. We employ a wide range of techniques in molecular biology as well as live cell imaging for the following studies;
- Intracellular signaling pathways activated by nanosecond pulsed electric fields
- Switching mechanism of two modes of cell death induced by nanosecond pulsed electric fields
- Live cell imaging of cellular responses to nanosecond pulsed electric fields
- Live cell imaging of DNA damage responses induced by pulsed laser microirradiation
1. Intracellular signaling pathways activated by nanosecond pulsed electric fields
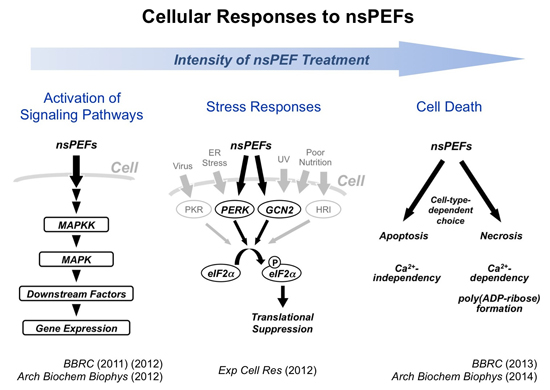
Nanosecond pulsed electric fields (nsPEFs) are recently regarded as an effective means for cancer therapy, but the molecular mechanism of their biological actions remains to be fully understood. We have been working to analyze intracellular events induced by nsPEFs and demonstrated that nsPEFs activate multiple signaling pathways. First, we found that nsPEFs induce phosphorylation of multiple protein components in representative MAPK pathways (JNK, p38, and ERK pathways) in human cells. Expression of immediate early genes, such as c-fos, c-jun, and Egr1, are also induced as a consequence of the MAPK activation by nsPEFs. Second, AMPK pathway is activated by nsPEFs. Third, nsPEFs act as a novel form of cellular stress and elicit transient suppression of general protein synthesis. We are further working to obtain a comprehensive view of the nsPEF-induced intracellular signaling network in human cells.
2. Switching mechanism of two modes of cell death induced by nanosecond pulsed electric fields
Apoptosis is a major mode of programmed cell death and is known to be induced by intense nsPEFs. We revealed that necrosis is another mode of cell death caused by intense nsPEFs. Many cell lines undergo necrosis instead of apoptosis. Chemical modification of cellular proteins, namely poly(ADP-ribose) formation, is associated with necrotic cell death caused by nsPEFs. Furthermore, the presence of extracellular calcium is a critical determinant of the sensitivity to nsPEFs and the choice of cell death modes. These findings indicate that the cell death mechanism activated by nsPEFs is more complicated than previously assumed. We currently seek to understand the detailed mechanism for switching two modes of cell death induced by nsPEFs.
3. Live cell imaging of cellular responses to nanosecond pulsed electric fields
In our lab, a state-of-the-art system for real-time imaging of living cells is introduced. This system allows us to use powerful imaging techniques, such as FRET and FRAP analyses. We are working to visualize various intracellular responses to nsPEFs to obtaine novel mechanistic insights into the nsPEF action.
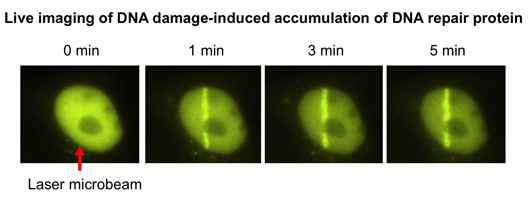
4. Live cell imaging of DNA damage responses induced by pulsed laser microirradiation
We have been working on the molecular mechanism of DNA damage responses in human cells. Our live imaging system is equipped with a generator of a UV laser microbeam and is able to irradiate a tightly defined area in a living cell. Using this system, DNA damage can be induced in a specific site in the nucleus at any time during microscopy, and dynamic behavior of nuclear proteins can be analyzed by time-lapse imaging and FRAP analysis. Using this powerful system, we are working to understand the functional roles of DNA repair factors and chromatin proteins in DNA damage responses.